At a glance
At a glance
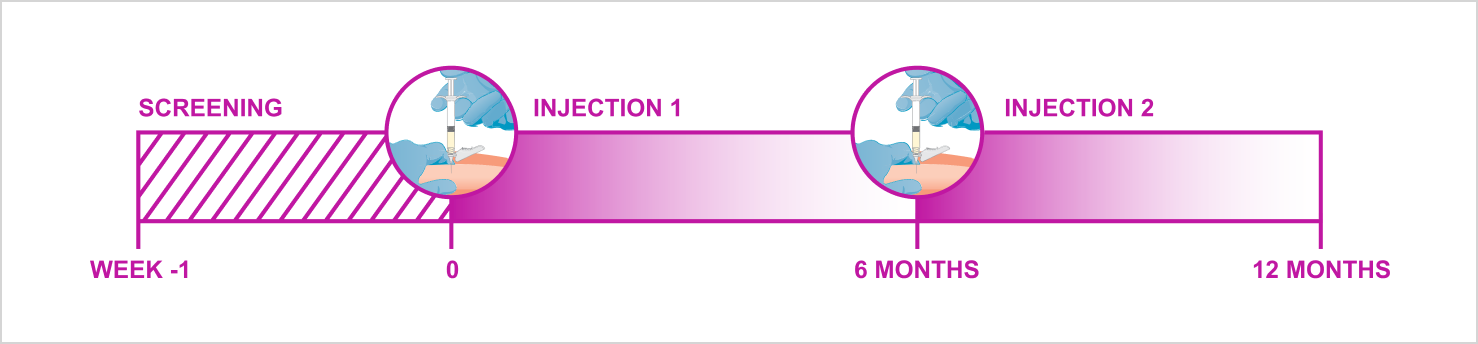
Fensolvi® is the first and only 6-month, subcutaneous (SC) injection of leuprolide acetate for CPP.[1]
Drug class
Fensolvi® (leuprolide acetate) for injectable suspension consists of leuprolide acetate delivered via a unique in-situ polymeric gel delivery system
Leuprolide acetate
- Leuprolide acetate is a gonadotropin releasing hormone (GnRH) agonist.
- It is the most commonly prescribed CPP treatment.[2]
Strength
45 mg
Delivery system
A single subcutaneous injection — with small injection volume and short 18 gauge needle — delivers 6 months of treatment.[3]
The innovative delivery system releases leuprolide acetate slowly over time as the polymer dissolves.[1]
Mechanism of action
GnRH agonist
Fensolvi is a GnRH agonist that inhibits the hypothalamic-pituitary-gonadal (HPG) axis, resulting in suppression of gonadotropin production, including luteinizing hormone, and ovarian and testicular production of estradiol and testosterone respectively.
Leuprolide acetate
- Leuprolide acetate is a potent synthetic nonapeptide analog of naturally occurring GnRH that inhibits pituitary gonadotropin secretion (luteinizing hormone and follicle stimulating hormone) when given continuously in therapeutic doses.
- Following an initial stimulation of gonadotropin releasing hormone receptors, chronic administration of leuprolide acetate results in downregulation of GnRH receptors, reduction in release of luteinizing hormone, follicle stimulating hormone and consequent suppression of ovarian and testicular production of estradiol and testosterone, respectively.
- This effect is reversible upon discontinuation of drug therapy.[4]
Indications
Fensolvi is indicated for the treatment of pediatric patients 2 years of age and older with central precocious puberty. Fensolvi treatment should be discontinued at the appropriate age of onset of puberty. Pubertal progression may resume within months and menses may begin from several months to more than 2 years after stopping treatment. [5]
References
- Fensolvi® (leuprolide acetate) for injectable suspension 45 mg Prescribing Information. Dublin 2, Ireland: Tolmar International, Ltd.; 11/2022.
- IMS Health. Accessed March 2020
- Klein K, Freire A, Gryngarten M, et al. Phase 3 trial of a small-volume subcutaneous 6-month duration leuprolide acetate treatment for central precocious puberty. J Clin Endo Metab. 2020;105(10):1-12.
- Fensolvi® (leuprolide acetate) for injectable suspension 45 mg Prescribing Information. Dublin 2, Ireland: Tolmar International, Ltd.; 2020. AND Engel JB, Schally AV. Drug Insight: clinical use of agonists and antagonists of luteinizing-hormone-releasing hormone. Nat Clin Pract Endocrinol Metab. 2007;3(2):157-167. AND Schally AV, Engel JB, Pinski J, Block NL. LHRH analogs. In: Handbook of Biologically Active Peptides. Elsevier Inc. 2013;531-540. doi:10.1016/b978-0-12-385095-9.00073-7
- Krishna KB, Fuqua JS, Rogol AD, et al. Use of gonadotropin- releasing hormone analogs in children: Update by an international consortium. Horm Res Paediatr 2019;91:357–372.